
There are many different types of batteries, and they are an invention that, believe it or not, dates back several thousand years.
What appears to be ancient batteries have been unearthed in what is now Iraq. These batteries were made of clay jars filled with vinegar. An iron rod surrounded by a copper cylinder was placed in the vinegar. These ancient cells typically produced 1-2 volts.
What ancient Iraqis would have done with 2V remains somewhat of a mystery, but the invention of the first “real” battery is credited to Alessandro Volta around 1800.
Why Volta and the not ancient Iraqis?
For one, no one knows who the original designer of the ancient battery was.
Secondly, it’s likely that Volta had at least some rudimentary understanding of how his battery worked, while no one’s sure if the ancient Iraqis did or if they even used it as a battery at all. The verdict on what exactly this artifact was intended for is still out.
Of course, Volta’s battery (and the ancient versions) were nothing like the batteries you’re used to working with today.
How Batteries Work
A modern-day battery consists of two separated dissimilar metals within an electrolyte. The whole package is enclosed in a container, usually either metal or plastic.
The electrolyte is usually an acid and causes a chemical reaction that pushes electrons from on plate or rod, through the electrolyte, to the opposite plate or rod. In other words, the reaction causes electrons to pass through the electrolyte as they are repelled from one plate and attracted to the other.
The rods/plates are the battery’s electrodes. One electrode is the anode and the other the cathode. When discharging, the anode is negative and the cathode positive. When charging a rechargeable battery, it’s the opposite.
The electrons are held in the negative electrode when nothing’s connected to the battery.
But, when something conductive, like an LED is place across its terminals the electrons flow through the circuit and do useful work.
Note that directly shorting the terminals of a battery can be dangerous, especially on larger batteries.
Real batteries have a finite shelf life and will eventually self-discharge even if never used.
Connecting Batteries Together
The way in which you connect batteries together (if you need more than one) depends on the needs of the device they’re powering.
Batteries in series will have voltages that will add up, so if you need more than 1.5 V this may be the way to go. The downside to this is that the total Ah capacity of the pack is equal to the capacity of one individual battery.
A group of batteries wired in parallel will produce more current. The voltage of the whole group of batteries will be the same as the voltage of any individual battery.
If you are concerned about wringing more amp-hours out of your batteries, this maybe the way to go. The total current (or Ah) that flows is the current (Ah) capability of any individual battery multiplied by the number of batteries in the group.
The figure below (borrowed from howstuffworks.com) illustrates this.
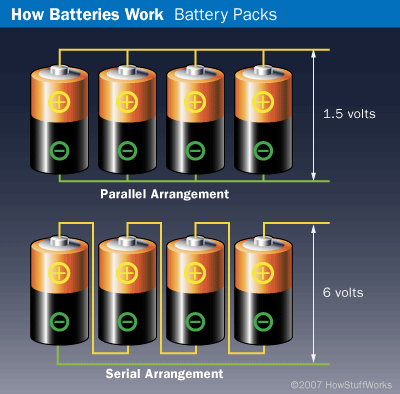
Battery Capacity
The amp-hour (Ah) is the unit of measure for battery capacity (C).
Recall that Coulombs are the unit of measure for charge (Q) and that one Coulomb is equal to the charge transferred in 1 second by 1 Amp of current. Put another way, 1 A = 1 Coulomb per 1 second. This simplifies to A (current) = Q (charge) divided by t (time in seconds). Therefore, current is equal to the amount of charge transferred per second. One Coulomb of charge contains 6.24 x 18 electrons.
Confused? The example below may help.
Ex. 1
Suppose 5 x 1019 electrons pass a point in a conductor in 2 seconds. How much current is that?
To figure this out, we first need to see how many Coulombs 5 x 1019 electrons is: (5 x 1019) / (6.24 x 1018) = 8 Coulombs.
Now, we simply divide the number of Coulombs by the time in seconds: Q/s = 8/2 = 4 A.
One Amp-hour is equal to 3600 Coulombs. This is because there are 3600 seconds in one hour.
From a practical standpoint, you likely won’t be counting electrons or measuring Coulombs.
So why all the fuss then?
As we said before, battery capacity is measured in Ah, and, as we’ve seen these lower-level units make up the Ah. The amp-hour is a unit that you may need to work with when designing things that run on batteries. Many of you may be familiar with the unit and what it actually means, but some of you may not, so I thought I’d peel back the layers for a moment.
Anyway, if I have a 20 Ah battery, it can supply 20 A for one hour or 10 A for 2 hours or 2 A for 10 hours and so on.
Here’s an example that can help you calculate the battery capacity you’ll need for a given project:
Ex. 2
Say you want to use a 300 Ah battery for your project and you know that your widget will draw 3 A. How many hours can you run your device before the battery dies?
All we need to do is divide the battery capacity by the amount of current your creation draws:
300 Ah/3 A = 100 hours of run time.
The 2 Types of Batteries
There are two main types of batteries: primary and secondary cells.
Become the Maker you were born to be. Try Arduino Academy for FREE!

Secondary cells are rechargeable, primary cells are not.
Because of space limitations, this post discusses primary cells from here on. A future post will go into secondary cells and their types and uses.
The type you end up picking will depend on the project.
We’re all familiar with D, C, AA, AAA and 9 V batteries that are cheap and readily available at most stores. These are examples of primary cells.
Some other types of primary batteries include button or coin cells, A23, 123 and others.
Primary Battery Cell Chemistry
The chemical reaction within a primary cell eventually dissolves the negative plate. Also, hydrogen gas bubbles form around the positive plate, causing a resistance between the two plates. This is known as the battery’s internal resistance. The addition of a depolarizer slows down the buildup of hydrogen gas bubbles, but with time the battery’s resistance still will increase as it discharges.
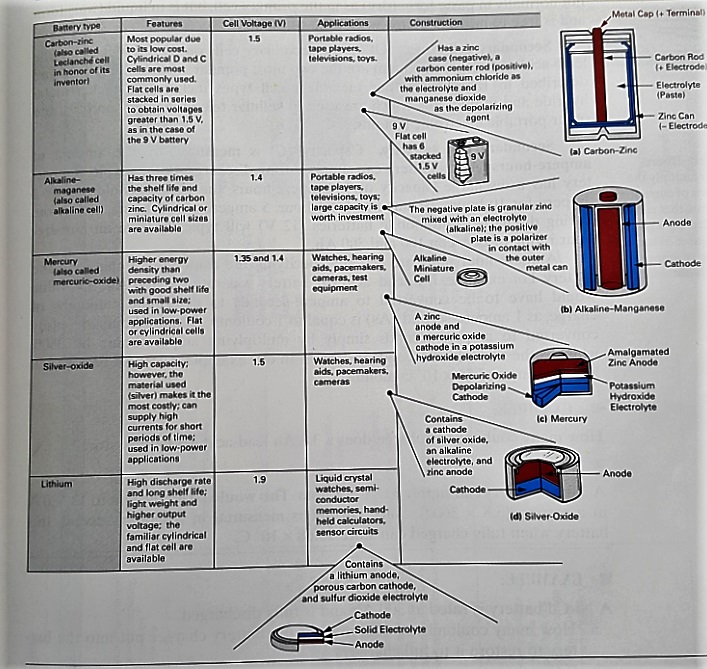
If you’re using a common battery size (such as AA or 9 V), you’re likely using one of three different chemistries: carbon-zinc, alkaline, or lithium.
Carbon-zinc batteries
These are the least expensive with a long shelf-life, but have the lowest capacity. They are good for low-drain devices like clocks, radios, and your TV remote. This is the original chemistry for batteries, dating back to the 1800s. The anode is zinc, the cathode is manganese dioxide, and the electrolyte is ammonium chloride or zinc chloride. The cathode contains powdered carbon to improve conductivity and for moisture retention.
Alkaline batteries
These have an even better shelf life and can last anywhere from 2-10x longer than carbon-zinc batteries (depending on the application), with only a small additional cost. This is why most household batteries today are of this type.
The cons are that standard alkalines don’t perform well in high-drain devices because they can’t supply current fast enough. More expensive, specialized alkaline batteries that perform a bit better in high-drain devices are available, but in cases like this you might as well use a rechargeable battery. They are also more prone to leaking and thus damaging electronics than carbon-zinc or lithium. Here are a few things you can do to reduce the likelihood of a leak.
- Do not try to recharge an alkaline battery
- Don’t mix fresh batteries with used ones
- Don’t mix alkaline batteries with other types
- Long exposure to high temperatures can cause leaking
- Don’t let dead batteries sit around inside your device
The cathode is composed of a manganese dioxide mixture, while the anode is a zinc powder. Alkaline batteries get their name from the potassium hydroxide electrolyte, which is an alkaline substance.
Lithium Batteries
Do not confuse Lithium batteries with lithium ion batteries, which are rechargeable.
Unlike carbon-zinc and alkaline batteries, lithium batteries work great in high-drain devices. They also boast the longest shelf life of the group, are light weight, and can operate in extreme temperatures ranging from -40 C up to +60 C.
The few cons are that they are the most expensive of the group and there is a small risk of explosion. Because of this risk you cannot send lithium batteries in the U.S. mail, nor can you check them with your baggage when flying.
One common type of Lithium battery uses lithium for the anode, iron disulfide for the cathode, and a lithium salt in an organic solvent blend as the electrolyte. However, per the Energizer PDF on lithium batteries, there are several different types of chemistries.
The document states that the term “lithium battery” refers to many different chemistries utilizing lithium as the anode but differing in cathode material, electrolyte, and construction. They may be classified in several ways, but one convenient method is by the cathode material and voltage. Using an iron disulfide cathode gives a battery with a nominal voltage of 1.5 volts. Most other lithium batteries are 3.0 volt systems using cathodes comprising either solids (manganese dioxide or carbon monofluoride) or highly toxic liquids (sulfur dioxide or thionyl chloride).
The chart below, taken from one of my old text books, summarizes most of the common types of batteries nicely. For some reason the chart does not include 1.5 V lithium batteries, but it does include lithium button cells.
Now we got some basic battery theory down and know more about common types primary cells. As I mentioned before, part two of this series will delve into types of secondary (rechargeable) batteries.
Stay tuned!
Update: part 2 about rechargeable batteries is here!
Become the Maker you were born to be. Try Arduino Academy for FREE!

Electronics Tips & Tutorials Sent Directly to Your Inbox

Submit your email & you'll get:
- Exclusive content that I don't put on the blog
- The checklist 10 mistakes all electronics enthusiasts make (& how to avoid them)
- And more!
This blog Types of Batteries & How They Work Part 1 helps me a lot
with my battery problems. I use a simple solutio for my
battery from here: [link removed] (or clik on name)
Kiss you all!